Complaint: AI Dermatologists App
Product: AI Dermatologist: Skin Scanner 17+
Diagnostic app claiming to detect melanoma and other skin conditions operating without proper MDR certification, missing essential documentation, and displaying fraudulent CE marking
Documented Violations
- Lack of Instructions for Use (IFU)
- Missing product label
- Absence of UDI registration
- No proper certification from notified body
- Fraudulent CE marking claims
- Violation of language requirements
- Operating as unregistered Class III medical device
Executive Summary
AI Dermatologists is a mobile application available on Apple App Store and Google Play Store that provides medical diagnoses for skin conditions, including melanoma detection. Despite functioning as a Class III medical device under MDR 2017/745, it operates without proper certification, lacks mandatory documentation, and displays fraudulent CE marking claims.
Product Information
Application Details
- Name: AI Dermatologist: Skin Scanner 17+
- Developer: Acina
- Platforms: iOS (Apple App Store), Android (Google Play Store)
- Website: https://ai-derm.com/
- Privacy Policy: https://ai-derm.com/privacy-policy
- Terms of Use: https://ai-derm.com/terms-of-use
Relevant Links
- AI Dermatologist on Apple App Store
- Acina’s Developer Page
- EUDAMED Database (No registration found)
- Web Archive of ai-derm.com (Independent verification of website content)
Claims and Intended Use
The application makes the following medical claims:
- “Our test can help you to detect melanoma”
- “An innovative prediagnostic app helps you to monitor and detect your skin health”
- “Experience the revolutionary AI-Dermatologist app, a cutting-edge solution to monitor your skin health and identify any potential skin conditions that require attention”
Functionality
Users upload images of skin lesions, and the application:
- Analyzes the image using AI algorithms
- Provides a specific diagnosis (e.g., “Benign Nevus”)
- Calculates risk percentages
- Recommends medical consultation
Disclaimer Contradiction
While the website includes a disclaimer stating “AI Dermatologist is not intended to perform diagnosis,” the actual functionality and marketing claims clearly position it as a diagnostic medical device.
Regulatory Classification
Medical Device Definition
According to MDR Article 2(1), the app qualifies as a medical device as it is:
“software intended by the manufacturer to be used for human beings for diagnosis, prevention, monitoring, prediction, prognosis of disease”
Risk Classification: Class III
The application must be classified as Class III (highest risk) based on:
- Rule 11 of MDR: Active devices intended to diagnose or monitor vital physiological processes
- MDCG 2019-11 Guidance: Software directly diagnosing critical conditions (melanoma)
- IMDRF/SaMD WG/N23: Software used by lay persons for serious conditions without professional support
Classification Table Reference (MDCG 2019-11, Annex III)
| State of Healthcare | Significance of Information: High (Treat or diagnose) |
|---|---|
| Critical situation | Class III (Category IV.1) |
Melanoma represents a critical healthcare situation, and the app directly diagnoses this condition, mandating Class III classification.
Documented Violations
1. Lack of Documentation (Article 10.11)
Violation: No Instructions for Use (IFU) or product label available Requirement: MDR Article 10.11 states:
“Manufacturers shall ensure that the device is accompanied by the information set out in Section 23 of Annex I”
Evidence:
- No IFU accessible within the app
- No IFU available on website
- Missing safety information
- No contraindications listed
- Absent side effect warnings
2. Missing Label (Article 10.11)
Violation: Complete absence of required label information Requirements per Annex I, Section 23:
- Manufacturer name and address
- Device version number
- UDI information
- Intended use statement
- Warnings and precautions
Status: None of these elements present
3. Lack of UDI (Article 27)
Violation: No Unique Device Identification Requirement: Article 10.7:
“Manufacturers shall comply with the obligations relating to the UDI system referred to in Article 27”
Evidence: No UDI visible or registered in EUDAMED
4. Lack of Certification (Article 52)
Violation: Operating without notified body approval Requirement: Class III devices require conformity assessment per Article 52 Evidence:
- No notified body number displayed
- No certification documentation available
- Extremely unlikely approval given other violations
5. Fraudulent CE Marking (Article 20)
Violation: Misleading CE marking presentation Evidence:
- CE mark displayed with “LL-C Certification” company name
- LL-C confirmed as ISO 13485 certifier, NOT a notified body
- No notified body identification number present
- Apparent attempt to mislead users about certification status
Legal Requirement (Article 20.5):
“CE marking shall be followed by the identification number of the notified body responsible for conformity assessment”
6. Language Requirements Violations
Violation: Documentation not available in required EU languages Requirement: MDR requires documentation in official languages of Member States where device is marketed Status: App available EU-wide but documentation only in English
Risk Assessment
Patient Safety Concerns
- Misdiagnosis Risk: False negatives for melanoma could delay critical treatment
- False Reassurance: Users may avoid professional consultation based on app results
- No Clinical Validation: Absence of clinical evidence documentation
- Unqualified Users: Designed for lay persons without medical training
Public Health Impact
- Potential for widespread harm given app store distribution
- Undermines legitimate medical device market
- Creates dangerous precedent for unregulated diagnostic tools
- Erodes public trust in digital health solutions
Supporting Evidence
Screenshot Analysis
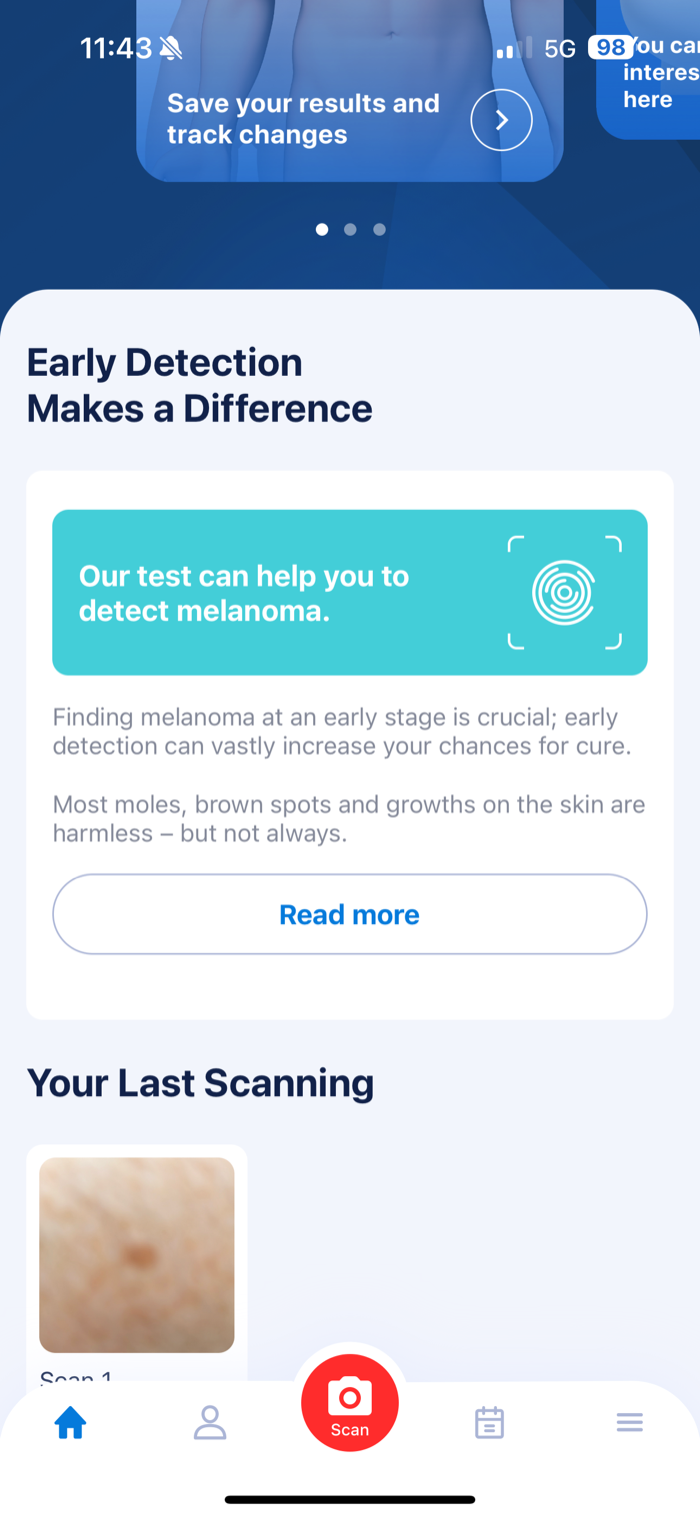
Screenshot 1: Main Screen
- Shows claim “Our test can help you to detect melanoma”
- Displays previous scanning results
- No regulatory information visible
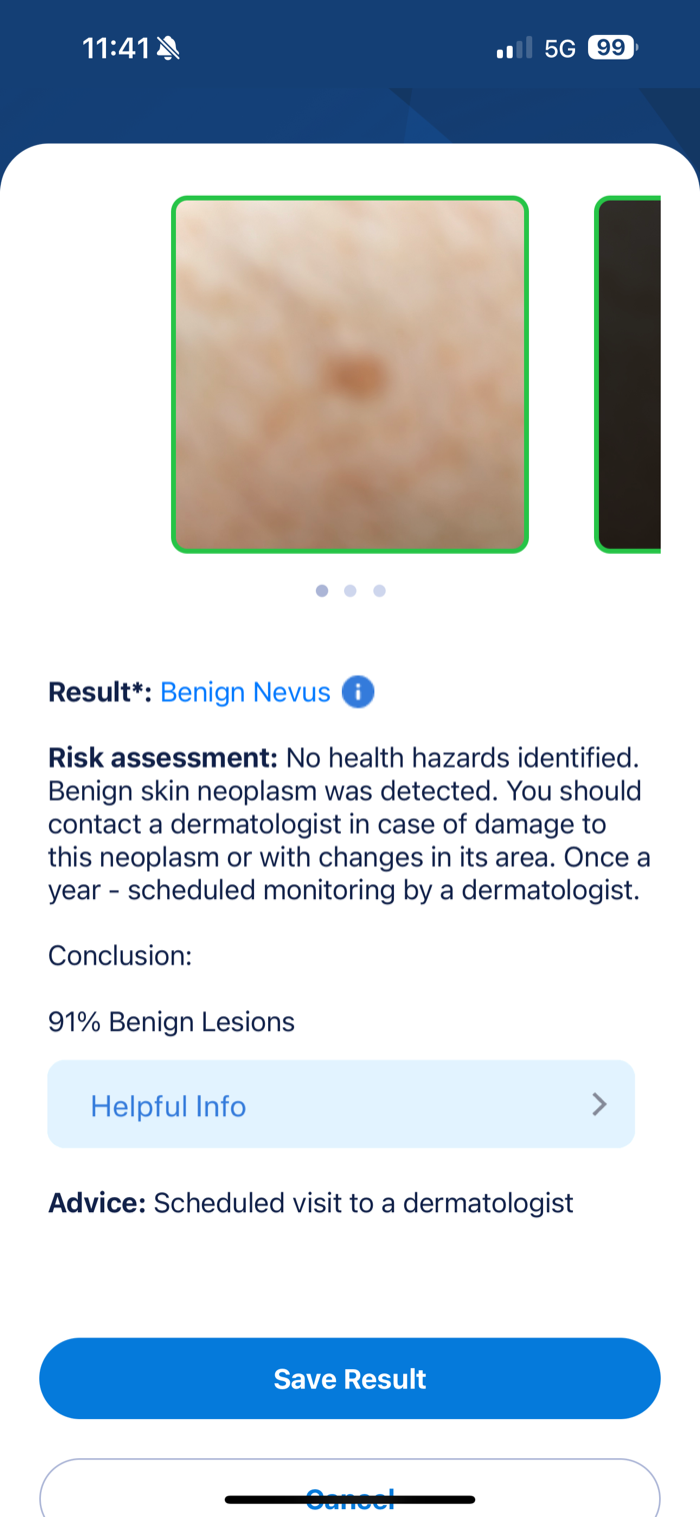
Screenshot 2: Diagnosis Result
- Provides specific diagnosis: “Benign Nevus”
- Shows risk percentage: “91% Benign Lesions”
- Includes medical advice: “Scheduled visit to a dermatologist”
- Clear diagnostic functionality demonstrated
Screenshot 3: CE Marking Display
- CE symbol prominently displayed
- “ISO-13485” text with LL-C Certification
- Misleading presentation suggesting proper certification
- No notified body number present
Regulatory Action Requested
Immediate Measures
- Order immediate cessation of EU market activities
- Remove from EU app stores (Apple, Google)
- Issue public safety notice
Investigation Requirements
- Full audit of company operations
- Review of all marketing materials
- Assessment of actual vs. claimed functionality
- Evaluation of patient harm reports
Enforcement Actions
- Financial penalties under Article 95
- Permanent market prohibition consideration
- Criminal investigation for fraudulent CE marking
- Coordination with other Member States
Legal Framework
Primary Violations
- Article 5: Placing device on market without conformity
- Article 10: General obligations of manufacturers (multiple breaches)
- Article 20: CE marking requirements
- Article 27: UDI system compliance
- Article 52: Conformity assessment procedures
- Annex I: General safety and performance requirements
Enforcement Basis
- Article 92: Justifies immediate corrective action
- Article 94: Supports market restriction/withdrawal
- Article 95: Enables proportionate penalties
Company Information
Manufacturer Details
- Company: Acina (per app store listing)
- Certification Claim: LL-C Certification (ISO 13485 only, not notified body)
- Website: ai-derm.com domain registration
- EUDAMED Status: No registration found
Conclusion
AI Dermatologists represents a severe violation of MDR 2017/745, operating as an unregistered Class III medical device with fraudulent certification claims. The application poses significant risk to public health through unvalidated diagnostic algorithms marketed directly to consumers.
Immediate regulatory intervention is essential to:
- Protect patients from potential misdiagnosis
- Maintain integrity of EU medical device framework
- Deter similar violations by other actors
- Demonstrate enforcement of MDR requirements
This case exemplifies the challenges posed by Software as Medical Device (SaMD) and the critical importance of robust regulatory oversight in the digital health sector.
Status Updates
October 15, 2025
Formal complaint submitted to relevant EU competent authorities
November 1, 2025
Complaint documentation updated with additional evidence and regulatory citations
This complaint was prepared by MD Watchdog’s team of volunteer regulatory professionals. All information is based on publicly available evidence and regulatory analysis.
Supporting Evidence